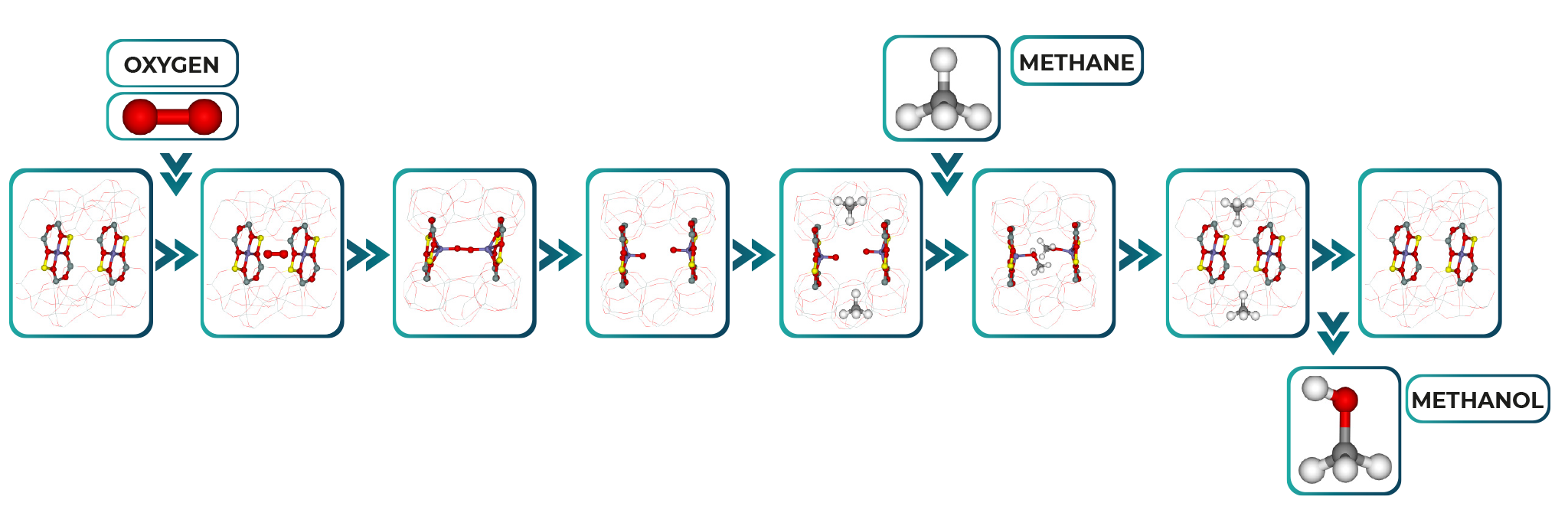
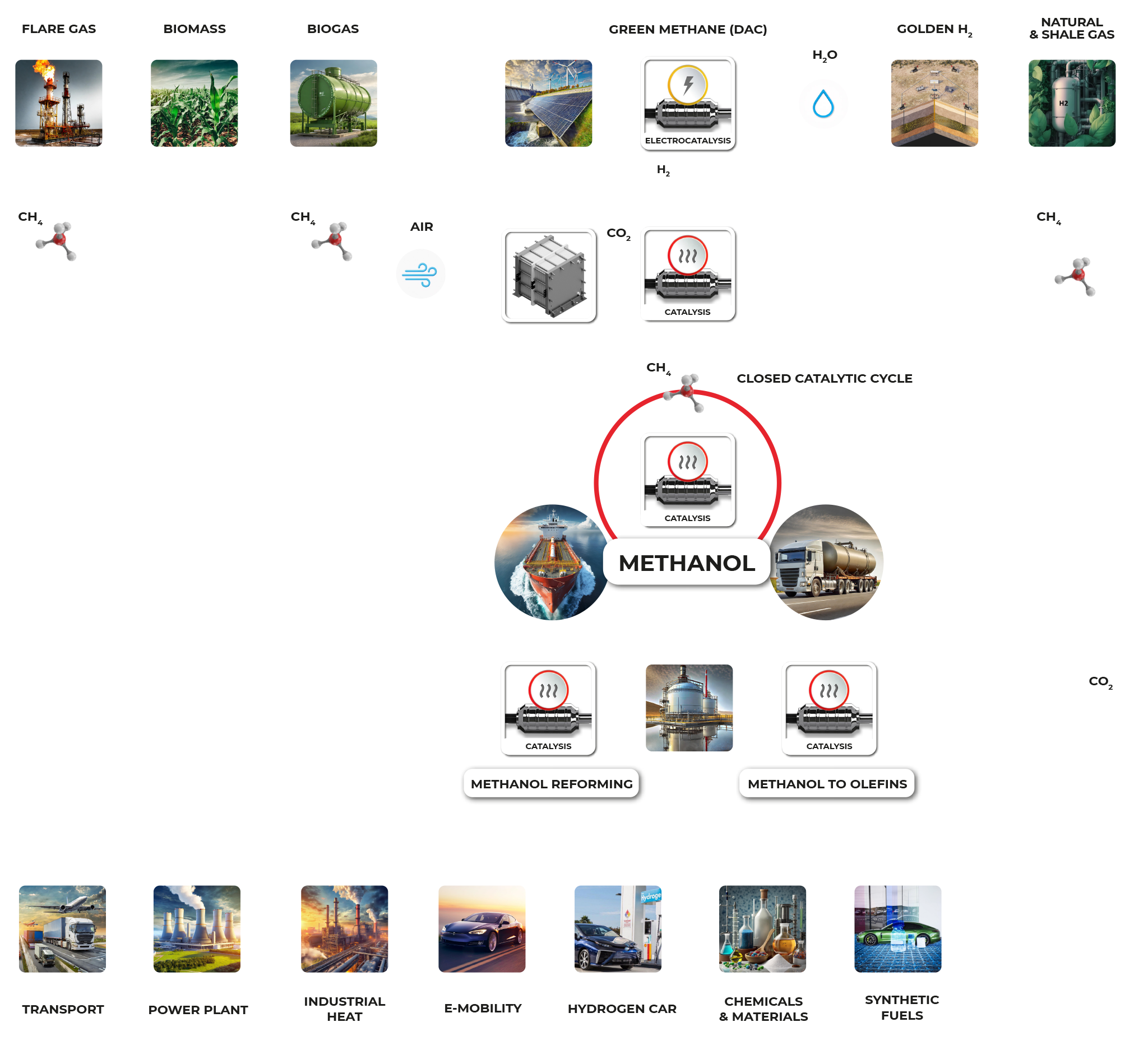
Methanol-derived products
Global methanol demand was approximately 90 million mt in 2023 and is expected to grow at a CAGR of approximately 4.5% p.a. This increase would be supported by a gradual shift to renewable methanol. The International Renewable Energy Agency, or IRENA, estimates the increase in methanol production is expected to see a progressive shift to renewable methanol, with an estimated annual production of 250 million mt of e-methanol and 135 million mt of biomethanol by 2050
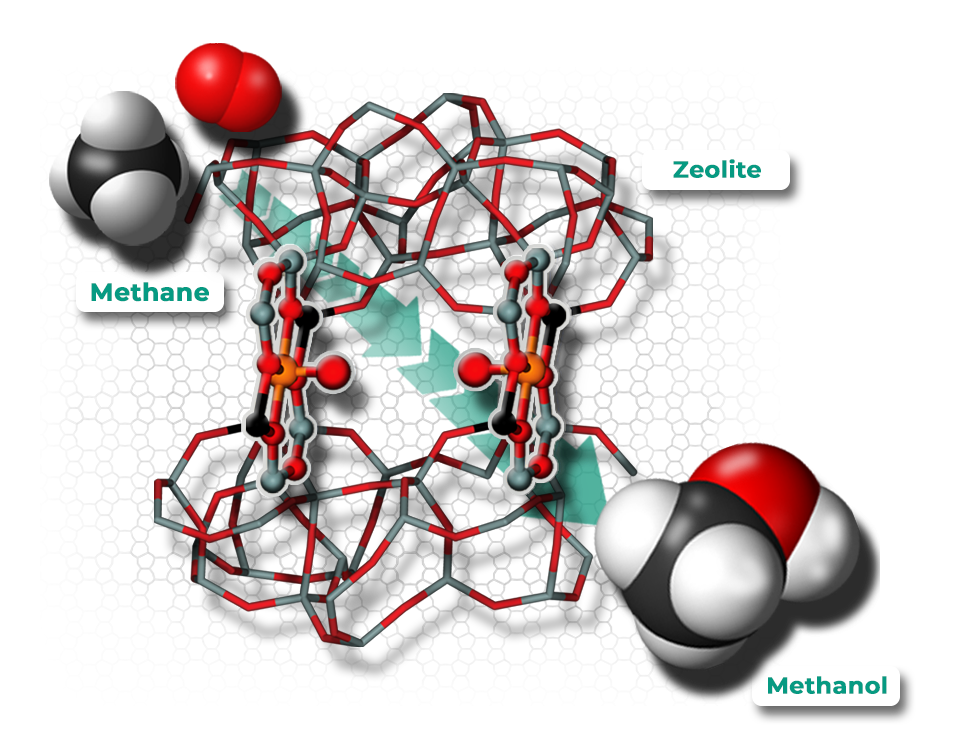











 Variability, flexibility and reliability of operations
Variability, flexibility and reliability of operations